What if the scent that makes you feel confident each morning, the fragrance that transforms your living space, or the subtle aroma in your favorite shampoo is actually a marvel of modern chemistry rather than a gift from nature? In a world where synthetic fragrances surround us—from our morning routines to our evening rituals—public perception remains largely shaped by misconceptions and assumptions. Many consumers instinctively believe that synthetic fragrances are inferior “artificial” substitutes for natural scents, viewing them as potentially harmful chemicals that lack authenticity and complexity. Others assume these laboratory-created compounds are cheap shortcuts that compromise both our health and the environment, dismissing them as toxic imposters in a world that increasingly values “natural” and “clean” products.
This comprehensive exploration challenges these assumptions by diving deep into the sophisticated science, surprising benefits, and complex realities of synthetic fragrances. We will uncover the intricate chemical processes behind their creation, examine their economic and performance advantages, while honestly addressing legitimate health and environmental concerns. Through detailed analysis of industry applications, regulatory challenges, quality standards, and comparisons with natural alternatives, this blog aims to provide you with the balanced, fact-based understanding needed to navigate the complex landscape of modern fragrance. By exploring both the innovations and controversies surrounding synthetic scents, you’ll discover why these laboratory-created molecules have become the backbone of the global fragrance industry—and what that means for your daily choices as a conscious consumer.

What Are Synthetic Fragrances?
Synthetic fragrances are complex chemical formulations created entirely in laboratory settings using artificial compounds and materials. Unlike natural fragrances derived from plants, flowers, and animal sources, synthetic scents are primarily made from petroleum-based chemicals, with 95% of their ingredients originating from these sources. These laboratory-created aromatic molecules can either mimic natural scents found in nature or introduce completely new fragrance profiles that don’t exist naturally.
The development of synthetic fragrances revolutionized the perfume industry, beginning in the 19th century when Paul Parquet first explored synthetic compounds by using coumarin to create Fougère Royal in 1882. Today, synthetic fragrances represent a sophisticated science that allows perfumers to create consistent, long-lasting scents that would be impossible to achieve through natural means alone.

Types of Synthetic Fragrances
Full Synthetic Fragrances
Full synthetic fragrances represent the pinnacle of laboratory chemistry, consisting entirely of artificial molecules created through advanced synthesis techniques. These formulations demonstrate the power of modern chemistry to create complex, long-lasting scents that outperform many natural alternatives.
Iso E Super is perhaps one of the most famous full synthetic fragrance molecules, discovered by International Flavors & Fragrances (IFF) in the 1970s. This cedarwood-like molecule creates a smooth, velvety woody scent that seems to hover close to the skin. It’s featured prominently in popular fragrances like Escentric Molecules Molecule 01, where it comprises 65% of the formula, and serves as a key component in many mainstream perfumes including Dior Fahrenheit and Hermès Terre d’Hermès.
Ambroxan (also known as Ambroxide) represents another breakthrough in synthetic fragrance chemistry. This molecule recreates the warm, amber-like qualities of natural ambergris without requiring the rare whale-derived ingredient. Ambroxan provides exceptional longevity and projects beautifully on skin, making it a cornerstone ingredient in modern perfumery. Notable examples include its prominent use in Maison Margiela Replica Beach Walk and as a base note in many luxury fragrances.
Calone revolutionized marine and aquatic fragrances when it was discovered. This synthetic molecule creates the distinctive “sea breeze” or “watermelon” effect found in many fresh, oceanic scents. Calvin Klein’s Escape and Davidoff Cool Water both rely heavily on calone to achieve their signature aquatic character that would be impossible to replicate using natural ingredients alone.
Applications: These full synthetic fragrances excel in commercial perfumes where consistency and longevity are paramount. They’re particularly valuable in air fresheners and household cleaning products because they maintain their scent profile even when mixed with harsh cleaning chemicals and provide consistent performance across different environmental conditions.

Semi-Synthetic Fragrances
Semi-synthetic fragrances represent a sophisticated hybrid approach that combines the best aspects of both synthetic and natural ingredients. This category allows perfumers to enhance natural materials with synthetic compounds or use synthetic ingredients to support and extend natural scents.
Enhanced Rose Formulations often combine natural rose oil or absolute with synthetic rose compounds like phenylethyl alcohol, citronellol, and geraniol. This approach allows perfumers to create fuller, more complex rose scents while controlling costs and ensuring consistency. Tom Ford’s Rose Prick, for example, uses this hybrid approach to create an intensely realistic yet long-lasting rose fragrance that maintains its character throughout the day.
Vanilla-Based Compositions frequently blend natural vanilla extract with synthetic vanillin and ethyl vanillin to achieve richer, more complex vanilla profiles. This combination provides the warmth and complexity of natural vanilla while adding the sweetness and projection that synthetic vanillins offer. Thierry Mugler Angel famously uses this approach, combining natural vanilla with synthetic components to create its distinctive gourmand profile.
Citrus Enhancements represent another common semi-synthetic approach. Natural citrus oils are notoriously short-lived, so perfumers often support them with synthetic citrus molecules like limonene synthetics and aldehydes. Acqua di Parma Colonia demonstrates this technique, using natural bergamot and lemon oils enhanced with synthetic citrus compounds to extend the fragrance’s freshness throughout the wear cycle.
Applications: Semi-synthetic fragrances are particularly popular in premium personal care products where brands want to highlight natural ingredients while ensuring product performance. They’re also common in niche perfumery, where perfumers seek to create unique interpretations of natural scents with enhanced longevity and projection.

Nature-Identical Compounds
The laboratory synthesis of Nature-identical compounds creates molecules that are chemically identical to those found in nature, but without requiring extraction from natural sources.
Synthetic Linalool is chemically identical to the linalool found naturally in lavender, rosewood, and many other plants. However, producing it synthetically allows for consistent quality and sustainable sourcing without depleting natural resources. This synthetic version appears in countless fragrances and personal care products, providing the same floral, slightly spicy character as its natural counterpart.
Laboratory-Created Benzyl Acetate replicates the sweet, jasmine-like molecule found naturally in jasmine flowers. Synthetic benzyl acetate provides the same olfactory profile as the natural version but offers superior consistency and cost-effectiveness. It’s a key component in many white floral fragrances and appears in products ranging from high-end perfumes to everyday soaps and shampoos.
Synthetic Eugenol mirrors the spicy, clove-like molecule found naturally in clove buds, cinnamon, and bay leaves. The synthetic version provides the same warm, spicy character while ensuring consistent potency and eliminating concerns about natural supply chain disruptions. It’s commonly used in oriental and spicy fragrances to add depth and warmth.
Vanillin Production represents perhaps the most successful example of nature-identical synthesis. While natural vanilla beans contain vanillin, over 99% of commercial vanillin is produced synthetically. This synthetic vanillin is chemically identical to natural vanillin but costs significantly less and provides consistent quality. It appears in everything from fine fragrances to food flavoring.
Applications: Nature-identical compounds are particularly valuable in applications where natural ingredients might be prohibitively expensive, seasonally unavailable, or ethically problematic. They’re extensively used in both luxury and mass-market fragrances, allowing brands to achieve natural scent profiles while maintaining cost-effectiveness and supply chain reliability.
Completely Artificial Scents
Completely artificial scents represent the most creative frontier of synthetic fragrance chemistry, producing olfactory experiences that have no natural counterpart. These molecules open entirely new categories of fragrance that expand beyond what’s possible in nature.
Cashmeran creates a unique musky-woody scent with cashmere-like softness that doesn’t exist in nature. This molecule provides a distinctive cozy, enveloping quality that has become essential in many modern fragrances. It appears prominently in fragrances like Maison Margiela By the Fireplace and contributes to the signature comfort factor in many contemporary perfumes.
Helional produces a fresh, marine-ozonic scent that evokes the smell of ironed laundry or sea spray. This completely artificial molecule created the foundation for the entire “clean” fragrance category that emerged in the 1990s. It’s a key component in fragrances like The Body Shop White Musk and appears in many fabric softeners and laundry detergents.
Norlimbanol generates a unique sandalwood-like scent that’s smoother and more linear than natural sandalwood, without the complexity that some find challenging in natural sandalwood oil. This molecule allows perfumers to achieve sandalwood effects without relying on increasingly rare natural sandalwood sources.
Synthetic Aldehydes (particularly C-10, C-11, and C-12 aldehydes) create sparkling, soapy, or fatty effects that became iconic in classic perfumes like Chanel No. 5. These molecules produce olfactory sensations that don’t exist naturally but have become synonymous with luxury and elegance in perfumery.
Fantasy Fruit Scents represent another category of completely artificial fragrances. Molecules that create convincing apple, pear, strawberry, or tropical fruit effects don’t exist naturally in extractable forms, yet synthetic chemistry has made these scents staples in modern perfumery and personal care products.
Applications: Completely artificial scents are particularly important in creating signature brand scents that can’t be easily replicated by competitors. They’re essential in the development of fantasy and conceptual fragrances that tell specific stories or evoke particular moods. These molecules are also crucial in functional applications like air fresheners, where unique, memorable scents help with brand recognition and market differentiation.

The Creation Process
Laboratory Synthesis Methods
Creating synthetic fragrances involves multiple sophisticated laboratory synthesis methods that transform basic chemical precursors into complex aromatic molecules. The process typically begins with petroleum-derived base chemicals that serve as building blocks for more elaborate fragrance compounds.
The most common synthesis methods include esterification reactions, where alcohols and acids combine to create fruity and floral esters like benzyl acetate (jasmine-like) and ethyl butyrate (pineapple notes). Aldol condensation reactions produce aldehydes that create sparkling, soapy effects essential in classic perfumery, such as the aldehydes that define Chanel No. 5’s signature opening.
Catalytic processes enable the creation of complex molecules like synthetic musks through cyclization reactions. For example, Galaxolide (HHCB) is produced through multi-step synthesis involving cyclization of specific organic precursors under controlled temperature and pressure conditions.
Advanced molecular engineering techniques allow chemists to modify existing natural molecules to enhance their performance characteristics. This might involve adding or removing functional groups to increase longevity, improve stability, or modify scent character while maintaining the desired olfactory profile.
After initial synthesis, crude fragrance compounds undergo extensive purification processes including distillation, crystallization, and chromatographic separation to achieve the purity levels required for commercial use. Modern synthetic fragrance molecules often exceed 95% purity, with some specialty compounds reaching 99%+ purity levels.
Quality control testing at this stage involves gas chromatography-mass spectrometry (GC-MS) analysis to verify molecular structure and identify any unwanted byproducts that could affect scent quality or safety profiles.

Chemist-Perfumer Collaboration Framework
The creation of successful synthetic fragrances requires seamless collaboration between analytical chemists who understand molecular behavior and synthesis pathways, and creative perfumers who possess the artistic vision and olfactory expertise to design appealing scent profiles.
Chemists bring technical expertise in molecular design, understanding how different chemical structures will translate into specific olfactory effects. They predict how molecules will interact, their volatility rates, their stability under various conditions, and their compatibility with different carrier systems.
Perfumers contribute creative vision, market knowledge, and refined olfactory sensibilities developed through years of training and experience. They understand how different molecules will blend harmoniously, how scents will develop over time on skin, and what combinations will appeal to target consumer segments.
The collaboration typically follows an iterative cycle where perfumers communicate their creative vision and performance requirements to chemists, who then synthesize candidate molecules or modify existing compounds to meet those specifications. This back-and-forth process continues through multiple rounds of refinement.
Prototype testing involves creating small batches of fragrance compositions that are evaluated for scent quality, performance characteristics, and stability. Both chemists and perfumers participate in these evaluations, with chemists focusing on technical performance and perfumers assessing aesthetic appeal.
Consumer testing integration brings additional feedback into the collaborative process, with both chemists and perfumers interpreting consumer responses to guide further refinement of formulations.

Longevity Engineering
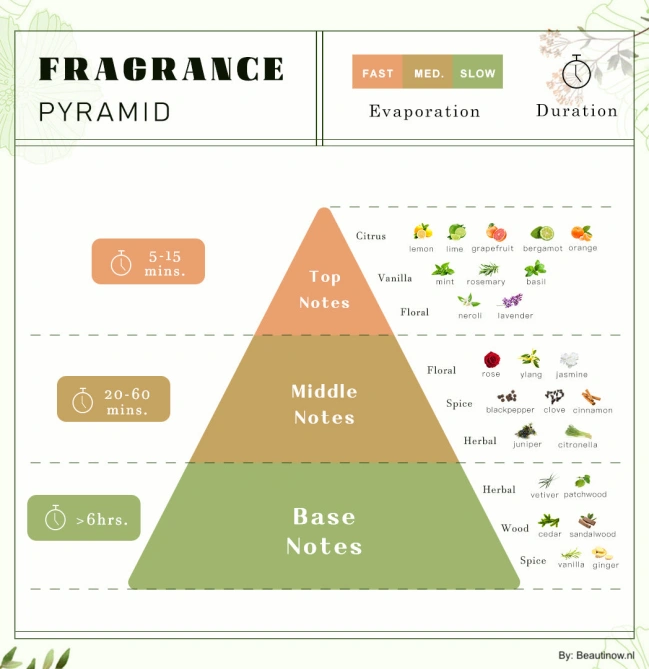
Achieving desired longevity requires careful selection of molecules with appropriate volatility characteristics. Perfumers and chemists work together to create fragrance pyramids with different evaporation rates: fast-evaporating top notes for immediate impact, medium-volatility heart notes for the main character, and slow-evaporating base notes for lasting power.
Substantivity factors are engineered through molecular design, with chemists creating molecules that bind effectively to skin and fabric surfaces. This involves understanding the lipophilic/hydrophilic balance of different compounds and how they interact with various surfaces.
Fixative integration involves incorporating synthetic molecules specifically designed to slow the evaporation of more volatile fragrance components, extending overall wear time without altering the intended scent profile.
Projection Optimization
Controlling projection requires understanding the diffusion characteristics of different molecules and how they interact in complex formulations. Chemists calculate optimal concentrations based on molecular weight, vapor pressure, and interaction effects between different compounds.
Sillage engineering involves creating formulations that project appropriately for their intended use, whether that’s intimate personal fragrances or broadly diffusing environmental scents. This requires precise balancing of volatile and less-volatile components.
Environmental adaptation considerations ensure that fragrances perform consistently across different temperature and humidity conditions, requiring chemists to understand how molecular behavior changes under varying environmental conditions.
Aesthetic Appeal Standards
Harmony and balance in fragrance composition require understanding how different chemical structures interact olfactorily. Chemists must understand not just individual molecule characteristics but also synergistic and antagonistic effects when molecules are combined.
Complexity development involves creating formulations that reveal different facets over time, requiring careful orchestration of molecules with different evaporation rates and interaction characteristics.
Uniqueness achievement often involves developing proprietary molecules that create distinctive scent signatures unavailable to competitors, requiring significant investment in original chemical research and synthesis.
Chemical Composition and Ingredients
Primary Chemical Components
The chemical foundation of synthetic fragrances relies heavily on petroleum-derived compounds. Key components include acetone, phenol, toluene, benzyl acetate, and limonene. These base chemicals serve as building blocks for more complex aromatic molecules that create the scents we recognize and enjoy.
Phthalates historically played a crucial role as scent stabilizers in synthetic fragrances, though their use has become controversial due to health concerns. These chemicals function as endocrine disrupting chemicals (EDCs) and have been linked to various health issues including elevated blood pressure, thyroid dysfunction, and diabetes. While many manufacturers have phased out phthalates from newer formulations, they may still be present in older products.
Synthetic musk compounds represent another significant category of fragrance chemicals, used as base notes in many perfume formulas. These compounds have raised particular concern as some varieties have been detected in human tissue and breast milk, with studies linking certain synthetic musks like musk ketone and tonalide to endocrine dysfunction and potentially increased breast cancer risk.
Carrier Substances
Carrier substances are the liquids that make concentrated fragrance oils usable and wearable. Think of them as the delivery system that transforms thick, concentrated aromatic molecules into the light, sprayable fragrances we use daily. These carriers do much more than simply dilute fragrances—they control how scents interact with your skin, spread into the air, and develop over time.
Choosing the right carrier can make or break a fragrance. Even the most beautiful aromatic blend will fail if it’s paired with the wrong carrier system, which is why professional perfumers pay careful attention to this crucial component.
Ethanol is the most common carrier in perfumery because it works exceptionally well for dissolving fragrance molecules and has a good safety profile. Its molecular structure can dissolve both water-loving and oil-loving fragrance compounds, making it perfect for complex synthetic fragrance blends. When you spray a fragrance, ethanol evaporates quickly, dispersing the scent molecules into the air for immediate projection.
This quick evaporation serves two purposes: it gives you an immediate burst of scent when you first apply the fragrance, and as it continues evaporating, it releases different fragrance notes over time, creating the classic top-to-base note progression that defines how a fragrance develops.
Isopropanol offers different evaporation speeds for specific needs. While less common than ethanol, it can be useful when perfumers want slower evaporation or when certain fragrance molecules work better with this type of alcohol. Denatured alcohol is simply ethanol with additives that make it undrinkable, allowing manufacturers to avoid alcohol taxes while keeping the performance benefits. The additives are carefully chosen so they don’t interfere with how the fragrance smells or performs.

How fast a carrier evaporates determines how your fragrance projects and how long it lasts. Fast-evaporating carriers like ethanol create strong initial projection but may fade more quickly, while slower carriers provide gradual release over longer periods. Perfumers must balance this based on the fragrance’s intended use—whether it should make an immediate impression or provide all-day presence. The interaction between carrier evaporation and individual fragrance molecules creates the complex patterns that define your fragrance experience.
Since carriers touch your skin directly, compatibility matters. Alcohol-based carriers can sometimes cause dryness or irritation, especially for people with sensitive skin or those who apply fragrance frequently. Alcohol’s astringent properties can strip natural skin oils and disrupt your skin’s protective barrier. The way carriers interact with your skin chemistry also affects how the fragrance develops throughout the day. As carriers evaporate and fragrance molecules mix with your skin’s natural oils and chemistry, the scent can change in ways that depend partly on the carrier used.
Recognizing that some people have alcohol sensitivities, the industry has developed alternatives. Silicone-based carriers spread smoothly and cause less skin irritation while providing different evaporation patterns than alcohol. These work well for leave-on products or people with alcohol sensitivities. Oil-based carriers offer another option, especially for niche or hypoallergenic fragrances. They typically evaporate more slowly and condition the skin better, though they change how the fragrance projects and develops compared to alcohol systems. The carrier oil can even add subtle background notes to the overall scent.
The carrier actively controls how your fragrance unfolds over time, influencing the transition between top, middle, and base notes. As it evaporates, it creates changing conditions that affect how different fragrance molecules are released and perceived. The carrier’s evaporation pattern must match the intended fragrance design. Fast-evaporating carriers work well for fragrances meant to develop quickly, while slower carriers better support fragrances designed to unfold gradually over many hours. Mismatched combinations can result in poor development or premature fading.
Carriers significantly influence projection (how far the scent travels) and sillage (the scent trail you leave behind). How quickly the carrier evaporates determines how fragrance molecules are released into the air and how they spread around you. Perfumers use different carrier characteristics to achieve specific projection profiles. Personal fragrances might use carriers for close-to-skin projection, while social fragrances might use carriers that create stronger projection and more noticeable sillage.
Complex Formulations
Modern synthetic fragrances are remarkably complex, often containing dozens to hundreds of individual chemical constituents. Fragrance formulations can include any combination of more than 3,500 different chemicals, each contributing to the overall scent profile. This complexity allows perfumers to create sophisticated compositions with distinct top, middle, and base notes that develop over time.
The proprietary nature of fragrance formulations means that manufacturers often protect their specific combinations as trade secrets, making it difficult for consumers to know exactly what chemicals they’re exposed to when using fragranced products.