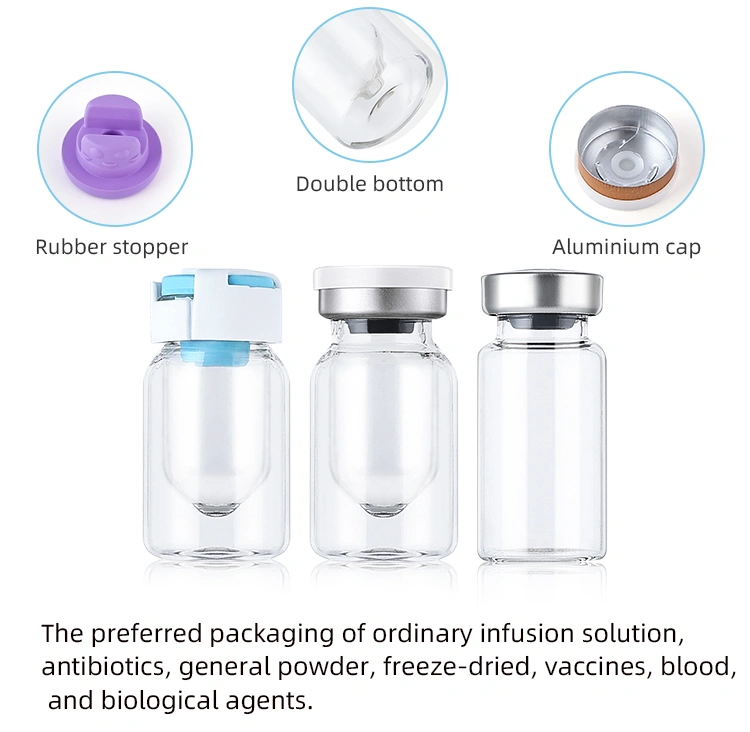
· LATEST DESIGN
· LATEST DESIGN
· LATEST DESIGN
· LATEST DESIGN
Jarsking covers all cosmetics,cannabis and perfume markets. Ask custom solutions here!
· EFFICIENCY+
· LATEST DESIGN
The world’s go-to cosmetic packaging factory for custom branding. Talk to Jarsking Team